Myeloproliferative neoplasms (MPN) are heterogenous clonal hematologic neoplasms where current therapies show limited disease-modification. We previously reported that the splicing factor, SF3B1, is mutated in 5-10% of MPN correlating with myelofibrotic progression in essential thrombocythemia. This adverse phenotype contrasts with SF3B1 mutationsin myelodysplasia where it is associated with milder disease. Moreover, SF3B1 K666 is the dominant hotspot in MPN in contrast to K700E in MDS (abstract #185043). The mechanism by which SF3B1 mutations accelerate myelofibrotic progression in JAK2V617F-mutated MPN is not understood and will be a key step towards the development of disease-modifying therapies.
To address this, we applied two complimentary single-cell multiomic methods to study 15 individuals (healthy donor [HD, n=5], JAK2V617F-single mutated [J+, n=5] and JAK2V617F- SF3B1K666double mutated MPN cases [JS+, n=5]). First, we characterised the cellular landscape of JS+ MPN using CITE-seq/10X genomics platform. Then, we analyzed SF3B1-mutant ( SF3B1mut) disease-driving hematopoietic stem and progenitor cells (HSPCs) and their associated aberrant splicing signatures using TARGET-seq, which permitted single-cell genotyping with allelic resolution and intra-patient comparison of mutant versus wild-type (wt) cells.
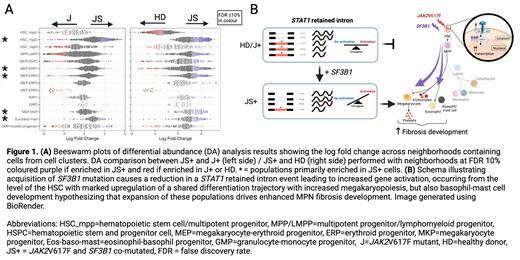
In an analysis of 109,498 cells, we observed abnormally expanded erythroid (ERP), megakaryocyte (MK) and unexpectedly, eosinophil-basophil-mast cell (EBM) progenitors in JS+ as compared with J+ or HD HSPCs (Fig 1A). Two HSC clusters were present, one of which (HSC_mpp2) principally comprised of JS+ cells and had a distinct molecular program (Fig 1A); upregulation in MK (eg. PF4, VWF) and EBM lineage signature genes (eg. TPSB2), interferon signaling genes but downregulation in apoptosis genes and canonical stemness markers (eg. CD133). These signatures suggest the JS+ driven HSC cluster may be MK/EBM-transcriptionally primed and may therefore, promote expansion of these abnormal progenitors which are implicated in driving fibrosis development.
Through genotyping of >5000 HSPCs, we resolved the clonal architecture of JS+ MPN, observing that in most cases double mutant cells were present and clonally dominant, with mutant SF3B1 the initiating event. Mutations arose from the HSC level and genotypes were equally distributed in the HSPC compartment. Highlighting the importance of single-cell analysis, and not apparent at the bulk level, JAK2V617F and SF3B1K666mutationsresided in separate clones for one case with a WHO fibrosis score 0 in contrast to the other 4 cases with double mutant cells and fibrosis scores 1-3. This reinforces the cooperative effect of thesemutationsto drive fibrosis.
We performed full-length transcriptomics of select HSPCs to study SF3B1mut-specific aberrant splicing events (ASE). In a global splicing analysis, more events were detected in JAK2V617F- SF3B1K666 double mutant than single mutant HSPCs. Retained intron (RI) events were the most prevalent ASE, followed by skipped exon and alternative 3' splice site events. We identified previously described SF3B1mut-specific ASE ( MAP3K7, ERGIC3 and SEPTIN6) confirming the validity of our dataset to study new ASE. Inter- and intra-patient comparison of SF3B1mut versus SF3B1wt cells uncovered a number of novel biologically relevant ASE, including two RI events in STAT1. These RI events were reduced in SF3B1mut HSPCs with the predicted consequence of increased STAT1 expression and activation, of direct relevance to MPN pathobiology since STAT1 is known to play a key role in enhancing megakaryopoiesis in MPN (Fig 1B). We validated these events using long-read Nanopore sequencing of single-cell cDNA libraries and have developed a cell model system with transient expression of SF3B1K666 mutation versus SF3B1wt in HEK293T to corroborate STAT1 ASE occurring as a direct consequence of mutant SF3B1. In further support of upregulated STAT1 activation, we observed upregulation of STAT1 downstream targets in abnormally expanded JS+ HSPCs.
In conclusion, this analysis resolved the clonal architecture and hematopoietic cellular composition of JS+ MPN, identifying abnormally expanded and transcriptionally primed HSPC populations with abnormal splicing of STAT1 enhancing JAK-STAT signaling, uncovering the mechanism by which these mutations promote an accelerated fibrotic phenotype.
Disclosures
O Sullivan:Morphosys: Honoraria; Novartis: Honoraria. Wen:AstraZeneca: Current Employment. Murphy:University of Oxford: Patents & Royalties: 2203947.3 . Simoglou Karali:BMS: Research Funding. Harrison:Galecto: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; AOP: Honoraria, Speakers Bureau; CTI: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Psaila:GSK: Honoraria; Blueprint Medicines: Honoraria; University of Oxford: Patents & Royalties: 2203947.3 ; Novartis: Speakers Bureau. Mead:Galecto: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Cofounder & equity holder, Research Funding; Karyopharm: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; University of Oxford: Patents & Royalties: 2203947.3 ; Relay Therapeutics: Consultancy, Speakers Bureau; GSK: Consultancy, Speakers Bureau; Roche: Research Funding; Incyte: Consultancy, Speakers Bureau; Sensyn: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Other: investigator for AbbVie sponsored trials, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal